medical mold standards
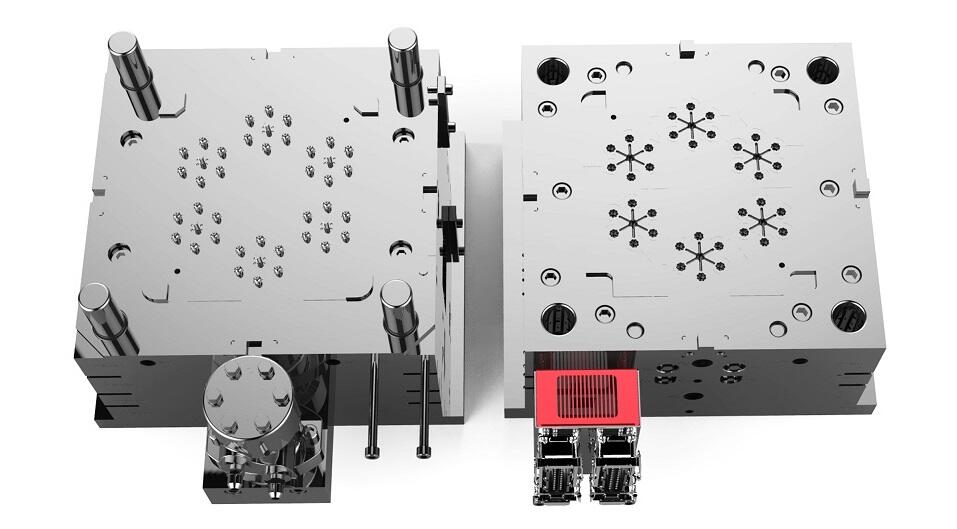
Medical mold standards represent a comprehensive framework of guidelines and specifications that ensure the consistent production of high quality medical devices and components. These standards encompass crucial aspects including material selection, design parameters, manufacturing processes, and quality control measures. The standards are meticulously developed to maintain strict compliance with regulatory requirements, ensuring patient safety and product reliability. Manufacturing facilities must adhere to specific cleanroom conditions, with controlled temperature, humidity, and particulate levels. The standards also dictate precise measurements, tolerances, and surface finish requirements, which are essential for producing medical devices that meet both functional and safety criteria. Advanced technologies such as Computer Aided Design (CAD) and Computer Aided Manufacturing (CAM) are integral to maintaining these standards, enabling precise reproduction and documentation of manufacturing processes. Quality control protocols, including regular testing and validation procedures, are embedded within these standards to ensure consistent product quality. The application of these standards extends across various medical sectors, from surgical instruments and diagnostic devices to implants and drug delivery systems.