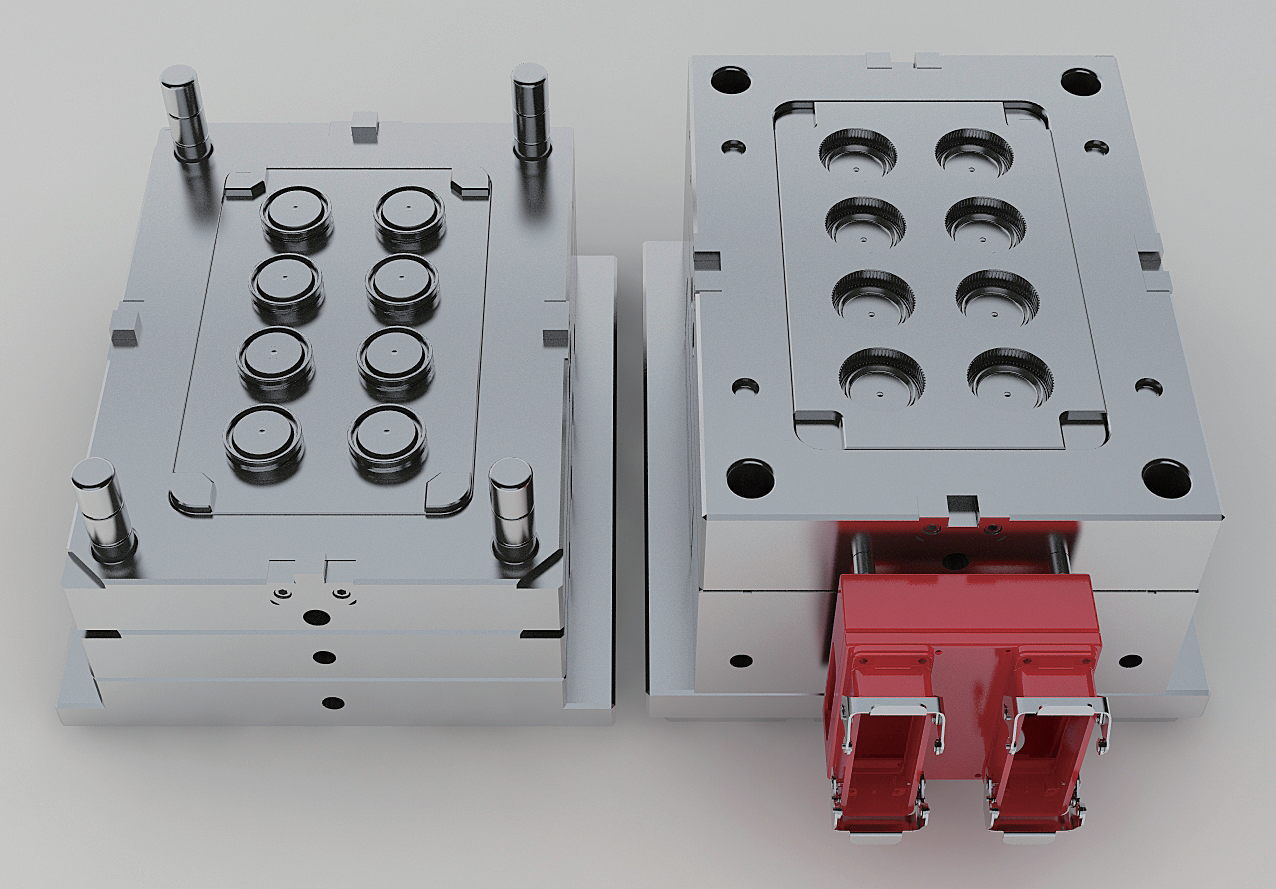
medical plastic injection molding
Medical plastic injection molding is a sophisticated manufacturing process that plays a crucial role in producing high-precision medical devices and components. This technology involves injecting molten plastic material into carefully designed molds under controlled conditions to create sterile, precise, and consistent medical parts. The process combines advanced engineering principles with stringent quality control measures to meet the demanding requirements of the healthcare industry. The technology utilizes specialized medical-grade polymers that are biocompatible and comply with FDA regulations. Key features include automated production capabilities, precise temperature control systems, and clean room manufacturing environments to ensure product sterility. The process enables the production of complex geometries with tight tolerances, essential for medical devices such as surgical instruments, diagnostic equipment components, and drug delivery systems. Modern medical injection molding facilities incorporate advanced quality monitoring systems, automated inspection processes, and documented validation procedures to maintain consistent product quality. The technology supports both high-volume production runs and specialized small-batch manufacturing, providing flexibility to meet varying market demands. Furthermore, the process allows for the integration of multiple materials and components, enabling the creation of sophisticated medical devices with enhanced functionality.