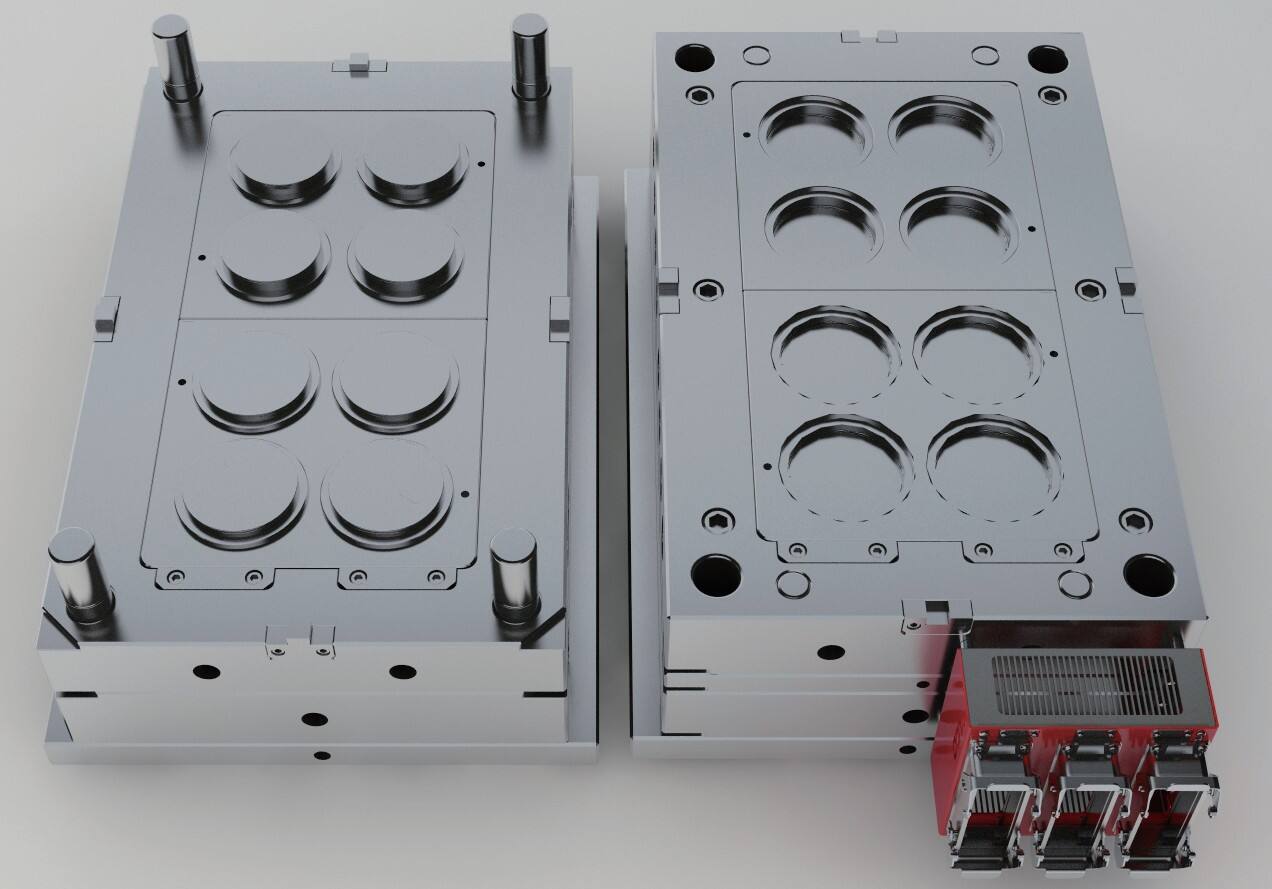
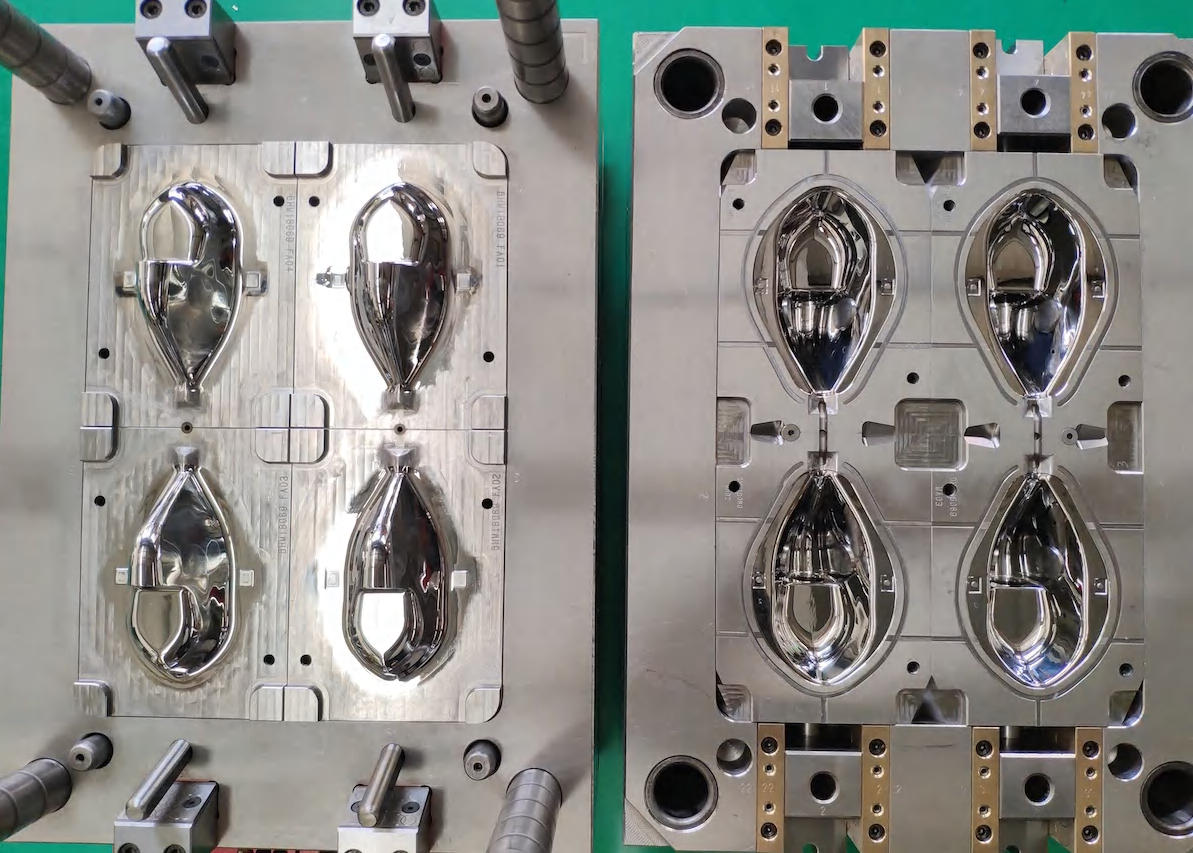
medical injection molding
Medical injection molding represents a sophisticated manufacturing process essential in producing high-precision medical devices and components. This advanced technology combines precision engineering with strict quality control protocols to create medical-grade plastic parts that meet rigorous healthcare industry standards. The process involves carefully heating specific medical-grade polymers until they reach a molten state, then injecting this material into custom-designed molds under precisely controlled pressure and temperature conditions. The technology ensures exceptional dimensional accuracy, consistently high quality, and the sterility required for medical applications. This manufacturing method is particularly valuable in producing complex medical components, from surgical instruments and diagnostic devices to drug delivery systems and implantable medical devices. The process accommodates various medical-grade materials, including thermoplastics like polycarbonate, polyethylene, and polypropylene, each selected for specific medical applications based on their unique properties. Modern medical injection molding incorporates advanced features such as clean room manufacturing environments, automated quality control systems, and real-time process monitoring to maintain the highest standards of product quality and patient safety. This technology has revolutionized medical device manufacturing by enabling large-scale production of complex medical components while maintaining strict adherence to regulatory requirements and industry standards.